-
 Agriculture
Agriculture
-
 Health-Care
Health-Care
-
 Environment
Environment
-
 Construction-Real-Estate
Construction-Real-Estate
-
 Tools-Hardware
Tools-Hardware
-
 Home-Garden
Home-Garden
-
 Furniture
Furniture
-
 Luggage-Bags-Cases
Luggage-Bags-Cases
-
 Medical-devices-Supplies
Medical-devices-Supplies
-
 Gifts-Crafts
Gifts-Crafts
-
 Sports-Entertainment
Sports-Entertainment
-
 Food-Beverage
Food-Beverage
-
 Vehicles-Transportation
Vehicles-Transportation
-
 Power-Transmission
Power-Transmission
-
 Material-Handling
Material-Handling
-
 Renewable-Energy
Renewable-Energy
-
 Safety
Safety
-
 Testing-Instrument-Equipment
Testing-Instrument-Equipment
-
 Construction-Building-Machinery
Construction-Building-Machinery
-
 Pet-Supplies
Pet-Supplies
-
 Personal-Care-Household-Cleaning
Personal-Care-Household-Cleaning
-
 Vehicle-Accessories-Electronics-Tools
Vehicle-Accessories-Electronics-Tools
-
 School-Office-Supplies
School-Office-Supplies
-
 Packaging-Printing
Packaging-Printing
-
 Mother-Kids-Toys
Mother-Kids-Toys
-
 Business-Services
Business-Services
-
 Commercial-Equipment-Machinery
Commercial-Equipment-Machinery
-
 Apparel-Accessories
Apparel-Accessories
-
 Security
Security
-
 Shoes-Accessories
Shoes-Accessories
-
 Vehicle-Parts-Accessories
Vehicle-Parts-Accessories
-
 Jewelry-Eyewear-Watches-Accessories
Jewelry-Eyewear-Watches-Accessories
-
 Lights-Lighting
Lights-Lighting
-
 Fabric-Textile-Raw-Material
Fabric-Textile-Raw-Material
-
 Fabrication-Services
Fabrication-Services
-
 Industrial-Machinery
Industrial-Machinery
-
 Consumer-Electronics
Consumer-Electronics
-
 Electrical-Equipment-Supplies
Electrical-Equipment-Supplies
-
 Electronic-Components-Accessories-Telecommunications
Electronic-Components-Accessories-Telecommunications
-
 Home-Appliances
Home-Appliances
-
 Beauty
Beauty
-
 Chemicals
Chemicals
-
 Rubber-Plastics
Rubber-Plastics
-
 Metals-Alloys
Metals-Alloys
- Masonry Materials
- Curtain Walls & Accessories
- Earthwork Products
- Fireproofing Materials
- Heat Insulation Materials
- Plastic Building Materials
- Building Boards
- Soundproofing Materials
- Timber
- Waterproofing Materials
- Balustrades & Handrails
- Bathroom & Kitchen
- Flooring & Accessories
- Tiles & Accessories
- Door, Window & Accessories
- Fireplaces & Stoves
- Floor Heating Systems & Parts
- Stairs & Stair Parts
- Ceilings
- Elevators & Escalators
- Stone
- Countertops, Vanity Tops & Table Tops
- Mosaics
- Metal Building Materials
- Multifunctional Materials
- Ladders & Scaffoldings
- Mouldings
- Corner Guards
- Decorative Films
- Formwork
- Building & Industrial Glass
- Other Construction & Real Estate
- Wallpapers/Wall panels
- HVAC System & Parts
- Outdoor Facilities
- Prefabricated Buildings
- Festive & Party Supplies
- Bathroom Products
- Household Sundries
- Rain Gear
- Garden Supplies
- Household Cleaning Tools & Accessories
- Lighters & Smoking Accessories
- Home Storage & Organization
- Household Scales
- Smart Home Improvement
- Home Textiles
- Kitchenware
- Drinkware & Accessories
- Dinnerware, Coffee & Wine
- Home Decor
- Golf
- Fitness & Body Building
- Amusement Park Facilities
- Billiards, Board Game,Coin Operated Games
- Musical Instruments
- Outdoor Affordable Luxury Sports
- Camping & Hiking
- Fishing
- Sports Safety&Rehabilitation
- Ball Sports Equipments
- Water Sports
- Winter Sports
- Luxury Travel Equipments
- Sports Shoes, Bags & Accessories
- Cycling
- Other Sports & Entertainment Products
- Artificial Grass&Sports Flooring&Sports Court Equipment
- Scooters
- Food Ingredients
- Honey & Honey Products
- Snacks
- Nuts & Kernels
- Seafood
- Plant & Animal Oil
- Beverages
- Fruit & Vegetable Products
- Frog & Escargot
- Bean Products
- Egg Products
- Dairy Products
- Seasonings & Condiments
- Canned Food
- Instant Food
- Baked Goods
- Other Food & Beverage
- Meat & Poultry
- Confectionery
- Grain Products
- Feminie Care
- Hair Care & Styling
- Body Care
- Hands & Feet Care
- Hygiene Products
- Men's Grooming
- Laundry Cleaning Supplies
- Travel Size & Gift Sets
- Room Deodorizers
- Other Personal Care Products
- Pest Control Products
- Special Household Cleaning
- Floor Cleaning
- Kitchen & Bathroom Cleaning
- Oral Care
- Bath Supplies
- Yellow Pages
- Correction Supplies
- Office Binding Supplies
- Office Cutting Supplies
- Board Erasers
- Office Adhesives & Tapes
- Education Supplies
- Pencil Cases & Bags
- Notebooks & Writing Pads
- File Folder Accessories
- Calendars
- Writing Accessories
- Commercial Office Supplies
- Pencil Sharpeners
- Pens
- Letter Pad/Paper
- Paper Envelopes
- Desk Organizers
- Pencils
- Markers & Highlighters
- Filing Products
- Art Supplies
- Easels
- Badge Holder & Accessories
- Office Paper
- Printer Supplies
- Book Covers
- Other Office & School Supplies
- Stationery Set
- Boards
- Clipboards
- Stamps
- Drafting Supplies
- Stencils
- Electronic Dictionary
- Books
- Map
- Magazines
- Calculators
- Baby & Toddler Toys
- Educational Toys
- Classic Toys
- Dress Up & Pretend Play
- Toy Vehicle
- Stuffed Animals & Plush Toys
- Outdoor Toys & Structures
- Balloons & Accessories
- Baby Food
- Children's Clothing
- Baby Supplies & Products
- Maternity Clothes
- Kids Shoes
- Baby Care
- Novelty & Gag Toys
- Dolls & Accessories
- Puzzle & Games
- Blocks & Model Building Toys
- Toddler Clothing
- Baby Clothing
- Kids' Luggage & Bags
- Arts, Crafts & DIY Toys
- Action & Toy Figures
- Baby Appliances
- Hobbies & Models
- Remote Control Toys
- Promotional Toys
- Pregnancy & Maternity
- Hygiene Products
- Kid's Textile&Bedding
- Novelty & Special Use
- Toy Weapons
- Baby Gifts
- Baby Storage & Organization
- Auto Drive Systems
- ATV/UTV Parts & Accessories
- Marine Parts & Accessories
- Other Auto Parts
- Trailer Parts & Accessories
- Auto Transmission Systems
- Train Parts & Accessories
- Universal Parts
- Railway Parts & Accessories
- Auto Brake Systems
- Aviation Parts & Accessories
- Truck Parts & Accessories
- Auto Suspension Systems
- Auto Lighting Systems
- New Energy Vehicle Parts & Accessories
- Auto Steering Systems
- Wheels, Tires & Accessories
- Bus Parts & Accessories
- Auto Performance Parts
- Cooling System
- Go-Kart & Kart Racer Parts & Accessories
- Air Conditioning Systems
- Heavy Duty Vehicle Parts & Accessories
- Auto Electrical Systems
- Auto Body Systems
- Auto Engine Systems
- Container Parts & Accessories
- Motorcycle Parts & Accessories
- Refrigeration & Heat Exchange Equipment
- Machine Tool Equipment
- Food & Beverage Machinery
- Agricultural Machinery & Equipment
- Apparel & Textile Machinery
- Chemical Machinery
- Packaging Machines
- Paper Production Machinery
- Plastic & Rubber Processing Machinery
- Industrial Robots
- Electronic Products Machinery
- Metal & Metallurgy Machinery
- Woodworking Machinery
- Home Product Manufacturing Machinery
- Machinery Accessories
- Environmental Machinery
- Machinery Service
- Electrical Equipment Manufacturing Machinery
- Industrial Compressors & Parts
- Tobacco & Cigarette Machinery
- Production Line
- Used Industrial Machinery
- Electronics Production Machinery
- Other Machinery & Industrial Equipment
- Camera, Photo & Accessories
- Portable Audio, Video & Accessories
- Television, Home Audio, Video & Accessories
- Video Games & Accessories
- Mobile Phone & Accessories
- Electronic Publications
- Earphone & Headphone & Accessories
- Speakers & Accessories
- Smart Electronics
- TV Receivers & Accessories
- Mobile Phone & Computer Repair Parts
- Chargers, Batteries & Power Supplies
- Used Electronics
- VR, AR, MR Hardware & Software
- Projectors & Presentation Equipments
- Other Consumer Electronics
- Cables & Commonly Used Accessories
- Computer Hardware & Software
- Displays, Signage and Optoelectronics
- Discrete Semiconductors
- Wireless & IoT Module and Products
- Telecommunications
- Connectors, Terminals & Accessories
- Development Boards, Electronic Modules and Kits
- Circuit Protection
- Sensors
- Isolators
- Audio Components and Products
- Integrated Circuits
- Power Supplies
- Relays
- RF, Microwave and RFID
- Electronic Accessories & Supplies
- Passive Components
- PCB & PCBA
- Air Quality Appliances
- Home Appliance Parts
- Heating & Cooling Appliances
- Small Kitchen Appliances
- Laundry Appliances
- Water Heaters
- Water Treatment Appliances
- Refrigerators & Freezers
- Personal Care & Beauty Appliances
- Major Kitchen Appliances
- Cleaning Appliances
- Second-hand Appliances
- Smart Home Appliances
- Other Home Appliances
- Energy Chemicals
- Inorganic Chemicals
- Basic Organic Chemicals
- Agrochemicals
- Admixture & Additives
- Catalysts & Chemical Auxiliary Agents
- Pigments & Dyestuff
- Coating & Paint
- Daily Chemicals
- Polymer
- Organic Intermediate
- Adhesives & Sealants
- Chemical Waste
- Biological Chemical Products
- Surface Treatment Chemicals
- Painting & Coating
- Chemical Reagents
- Flavor & Fragrance
- Non-Explosive Demolition Agents
- Other Chemicals
- Custom Chemical Services
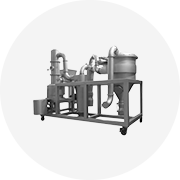
Understanding Vacuum Evaporator Use
Operational Principles
Vacuum evaporators work by lowering the pressure within the evaporation chamber. This reduced pressure lowers the boiling point of the liquid, allowing evaporation to occur at a significantly lower temperature. This is particularly beneficial when dealing with heat-sensitive materials, preventing degradation or decomposition. The reduced boiling point also translates to lower energy consumption compared to atmospheric evaporators.
The process typically involves feeding the liquid into the evaporator, where it's heated. The generated vapor is then removed by a vacuum pump, preventing it from condensing back into the liquid. The concentrated liquid is subsequently collected, while non-condensable gases are vented. The effectiveness of the vacuum is critical; insufficient vacuum will lead to inefficient evaporation, while excessive vacuum can cause unwanted foaming or bumping.
Applications Across Industries
The versatility of vacuum evaporators makes them indispensable in numerous industries. In the pharmaceutical industry, they're used to concentrate sensitive extracts and remove solvents from drug formulations. The food industry utilizes them to concentrate fruit juices, dairy products, and other liquids, often preserving flavors and nutrients better than high-temperature methods.
Chemical manufacturers employ vacuum evaporators for concentrating solutions, recovering solvents, and crystallizing products. Wastewater treatment plants use them to remove water from sludge, reducing volume and facilitating disposal. The specific design and configuration of the evaporator often depend on the particular application and the properties of the liquid being processed.
Advantages Over Conventional Evaporators
The primary advantage of vacuum evaporation is the lower boiling point, which minimizes thermal degradation and energy consumption. This is particularly significant for heat-sensitive materials where conventional evaporation at atmospheric pressure would lead to undesirable changes in quality or yield. This lower temperature operation also extends the lifespan of the equipment.
Furthermore, vacuum evaporation allows for the processing of liquids with high boiling points, which might be difficult or impossible to evaporate at atmospheric pressure. The controlled evaporation process offers better quality control and allows for precise concentration levels. The ability to handle volatile solvents safely is another important advantage, improving worker safety and minimizing environmental risks.
REPORT